Global Clinical Trials Market Soars: Innovations and Investments Reshape the Future of Drug Development 2025
The Global Clinical Trials Market is expected to reach at a CAGR of 7.4% during the forecast period 2025-2033.
The Global Clinical Trials Market is expanding rapidly, driven by rising R&D investments, innovative therapies, and increasing demand for faster, safer drug development.”
AUSTIN, TX, UNITED STATES, August 27, 2025 /EINPresswire.com/ -- Overview of the Market:— DataM Intelligence
The Global Clinical Trials Market is a pivotal component of the healthcare and pharmaceutical industries, facilitating the development of new therapies and medical devices through structured research studies. The market was valued at around USD 70.97 billion in 2024, and it is expected to reach USD 143.46 billion by 2033, increasing at a 7.4% CAGR. This growth is primarily driven by the increasing prevalence of chronic diseases, advancements in medical technology, and the globalization of clinical research.
To Download Sample Report Here: https://www.datamintelligence.com/download-sample/clinical-trials-market
The Market's expansion is further fueled by the rising demand for personalized medicine, the adoption of decentralized trial models, and the increasing reliance on Contract Research Organizations (CROs) for conducting trials. North America currently leads the market, holding a significant share due to its robust healthcare infrastructure and substantial investment in research and development.
Key Highlights from the Report:
The global clinical trials market was valued at USD 70.97 billion in 2024 and is projected to reach USD 143.46 billion by 2033, growing at a CAGR of 7.4%.
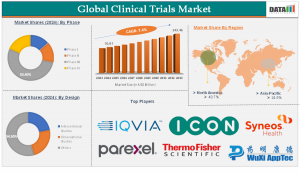
Phase III trials dominate the market, accounting for over 52% of total revenue in 2024.
Oncology remains the leading therapeutic area, representing approximately 26% of the market share in 2024.
North America accounted for 44.19% of the global market in 2024, with the Asia Pacific region expected to rise at a CAGR of 7.16% during the projected period.
The interventional study design segment captured 70.50% of the market share in 2024.
The increasing adoption of decentralized and hybrid trial models is enhancing patient recruitment and retention rates.
Market Segmentation:
By Phase
The clinical trials market is segmented into four phases:
Phase I focuses on determining the safety and dose of a medicine in a limited group of healthy participants.
Phase II: Assesses the efficacy and side effects of a medicine in a broader population of patients.
Phase III involves confirming the drug's effectiveness, monitoring adverse effects, and comparing it to regularly used treatments in a broad patient population.
Phase IV: Involves post-marketing surveillance to detect any rare or long-term adverse effects.
Among these, Phase III trials dominate the market due to their large scale and critical role in the drug approval process.
By Study Design
The market is categorized based on study design into:
Interventional Studies: Participants receive specific interventions according to the research plan.
Observational Studies: Researchers observe participants without intervening.
Expanded Access Studies: Offer investigational medications to patients outside of clinical trials.
Interventional studies hold the largest market share, reflecting their predominant use in clinical research.
By Indication
Key therapeutic areas in clinical trials include:
Oncology: Leading the market with approximately 26% share.
Cardiology: Focuses on heart-related conditions.
Neurology: Covers disorders of the nervous system.
Infectious Diseases: Includes trials for diseases caused by pathogens.
Endocrinology: Deals with hormone-related conditions.
Oncology remains the most prevalent indication due to the high demand for cancer treatments.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=clinical-trials-market
By Region
Regional dynamics significantly influence the clinical trials market:
North America: Dominates the market with advanced healthcare infrastructure and substantial R&D investments.
Europe: Faces challenges due to complex regulatory processes, leading to a decline in clinical trial activities.
Asia Pacific is emerging as a clinical trial hub due to lower costs and a larger patient pool.
Latin America and Middle East & Africa: Show potential for growth with increasing healthcare investments.
Market Dynamics:
Market Drivers
Rising Prevalence of Chronic Diseases: An increase in conditions like cancer, diabetes, and cardiovascular diseases necessitates the development of new treatments.
Advancements in Medical Technology: Innovations such as AI, wearable devices, and electronic data capture are streamlining clinical trial processes.
Globalization of Clinical Research: Expanding clinical trials into emerging markets offers diverse patient populations and cost-effective solutions.
Supportive Government Initiatives: Policies and funding are encouraging clinical research, particularly in oncology and rare diseases.
Market Restraints
High Costs of Clinical Trials: The expenses associated with conducting clinical trials can be prohibitive, especially in early phases.
Regulatory Challenges: Complex and varying regulations across regions can delay trial initiation and approval processes.
Recruitment and Retention Issues: Difficulty in enrolling and retaining participants can impact trial timelines and outcomes.
Market Opportunities
Decentralized and Hybrid Trials: These models improve patient access and retention by reducing the need for site visits.
Emerging Markets: Countries like India and China offer large, diverse patient populations and cost advantages.
Personalized Medicine: Tailoring treatments to individual genetic profiles opens new avenues for clinical research.
Real-World Evidence: Utilizing data from real-world settings enhances the relevance and applicability of trial results.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Frequently Asked Questions (FAQs)
How Big is the Clinical Trials Market?
What is the Projected Growth Rate of the Clinical Trials Market?
Who are the Key Players in the Global Clinical Trials Market?
What is the Market Forecast for 2033?
Which Region is Estimated to Dominate the Clinical Trials Market through the Forecast Period?
Company Insights:
Key players in the clinical trials market include:
IQVIA
ICON plc
Parexel
PPD (Thermo Fisher Scientific)
Syneos Health
WuXi AppTec
PSI
Medpace
Lindus Health
Precision for Medicine
Charles River Laboratories International, Inc.
Syneos Health, Inc.
Pharmaron Beijing Co., Ltd.
Clinipace (Caidya)
Celero
SGS SA
Chiltern International Ltd (Laboratory Corporation of America, LabCorp/Covance)
Eli Lilly and Company
Novo Nordisk A/S
Pfizer.
Recent Developments:
USA:
In April 2025, the FDA laid off approximately 3,500 employees, including key officials overseeing drug approvals and biologics. This restructuring has led to significant backlogs in regulatory guidance, delaying clinical trials and adding financial strain on biotech companies.
On August 25, 2025, AbbVie announced a deal worth up to $1.2 billion to acquire rights to bretisilocin, an experimental depression drug from Gilgamesh Pharmaceuticals. This move marks AbbVie's entry into the psychedelic-based treatment market.
Japan:
As of August 21, 2025, Takeda Pharmaceuticals revealed plans to launch global clinical trials in India, aiming to fast-track the introduction and availability of their innovative drugs in the Indian market.
On June 30, 2025, Japan's Ministry of Health, Labor, and Welfare released its 2025 clinical trials strategy report, advocating for the use of single institutional review boards (IRBs) to enhance the international competitiveness of clinical research.
Conclusion:
The Clinical Trials Market is poised for significant growth, driven by advancements in medical technology, an increasing prevalence of chronic diseases, and the globalization of research activities. While challenges such as high costs and regulatory complexities exist, opportunities in decentralized trials, emerging markets, and personalized medicine present avenues for innovation and expansion. Stakeholders across the healthcare and pharmaceutical sectors must navigate these dynamics to capitalize on the evolving landscape of clinical research.
Related Reports:
Anti-Obesity Drugs Market:
Biosimilars Market
Sai Kiran
DataM Intelligence 4Market Research
+1 877-441-4866
Sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.